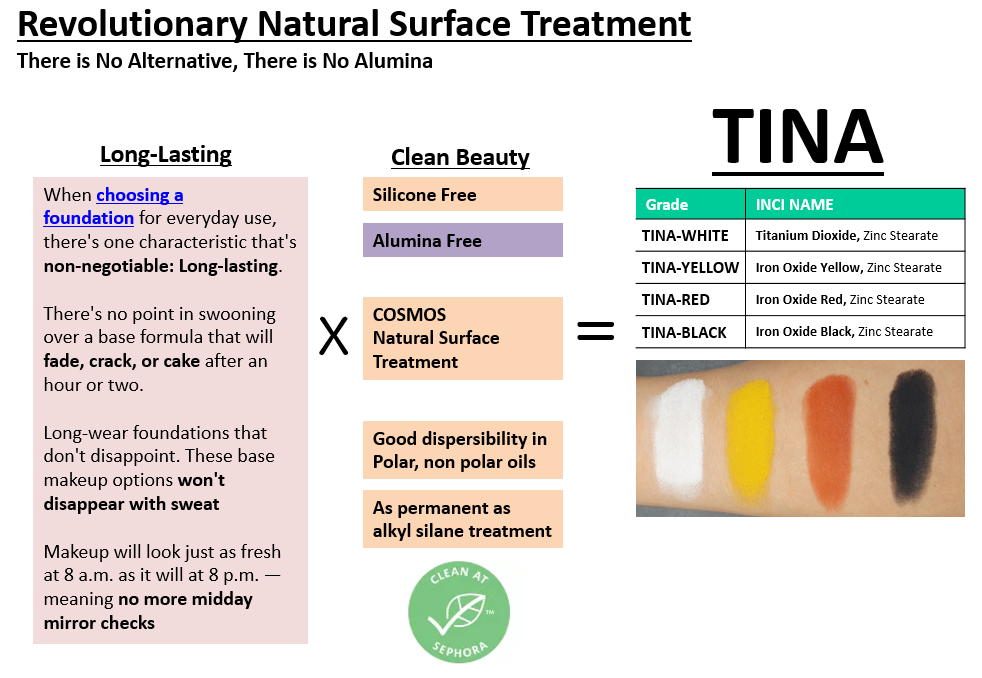
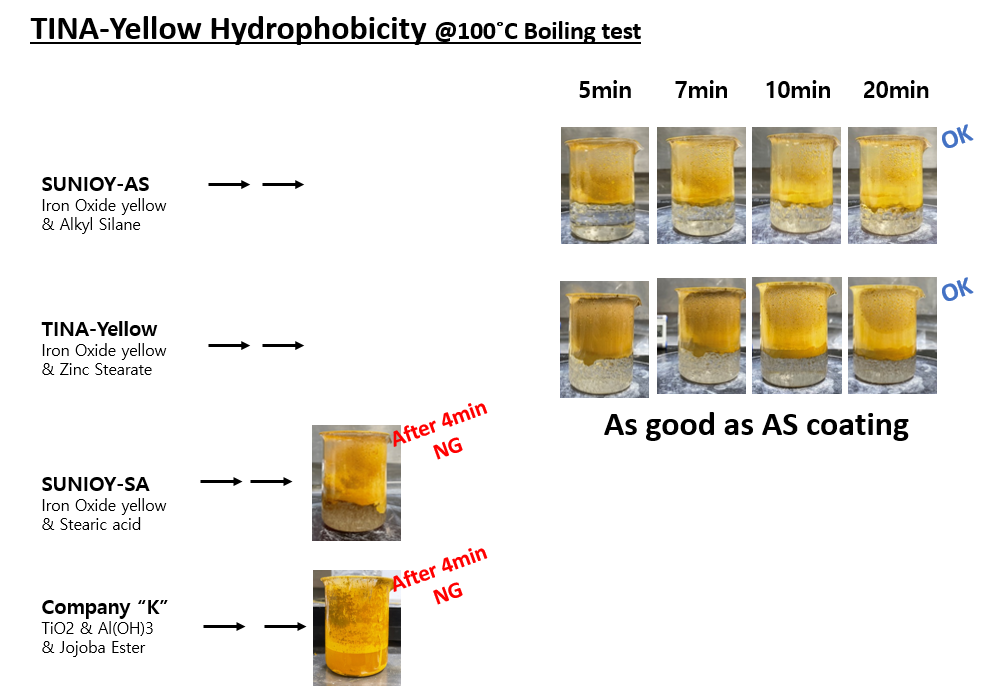
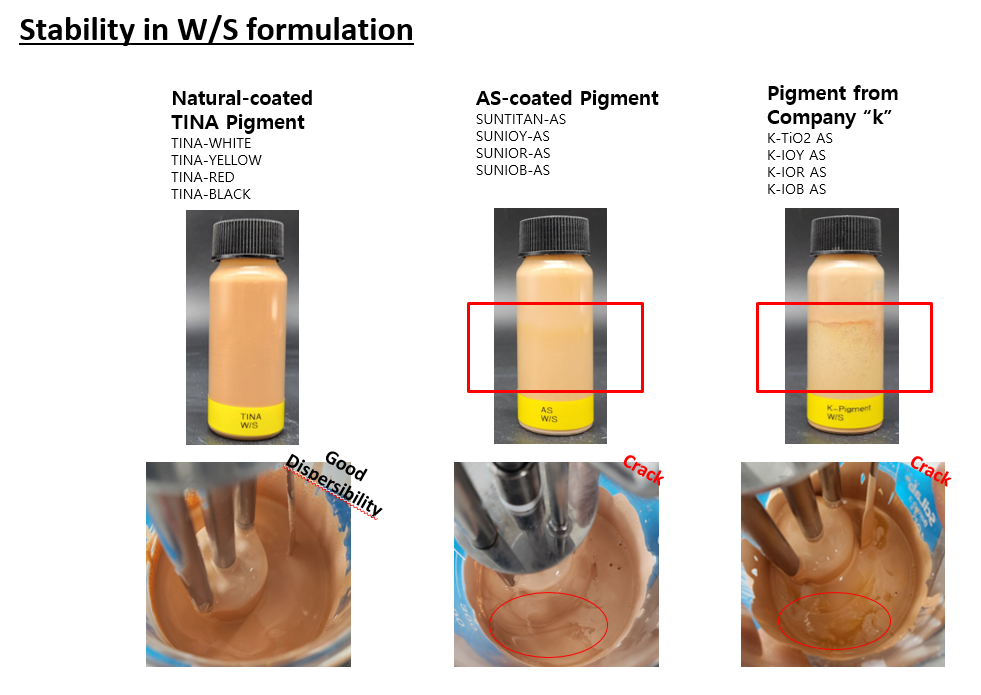
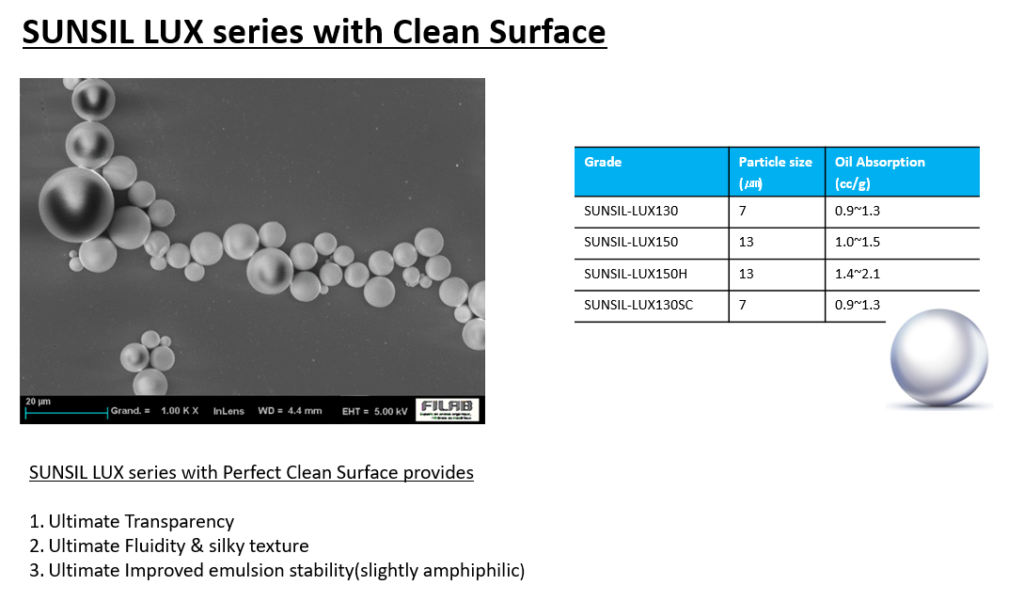
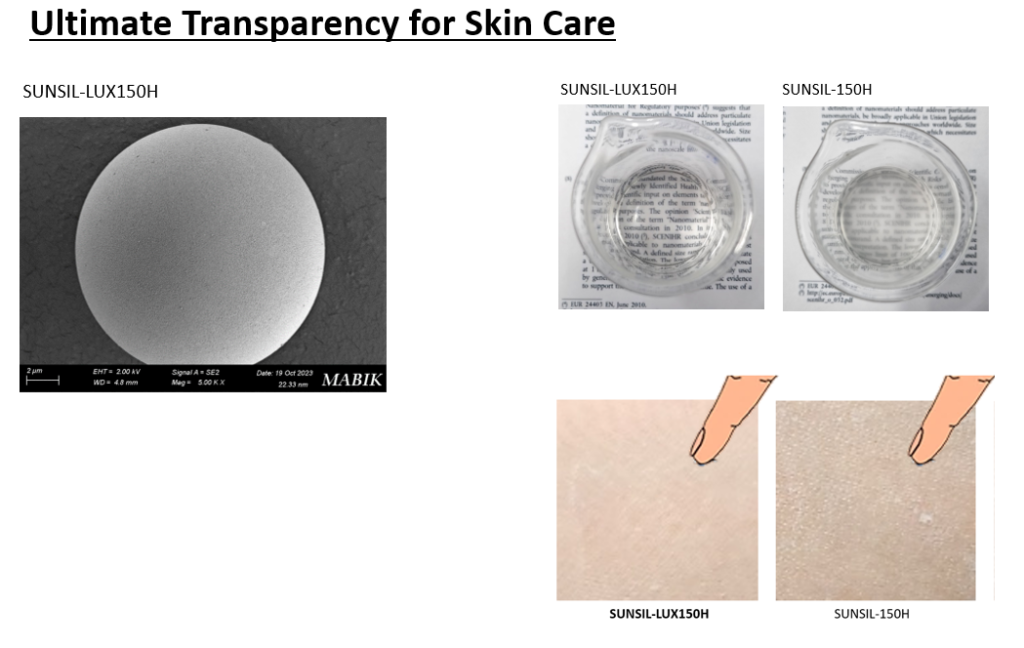
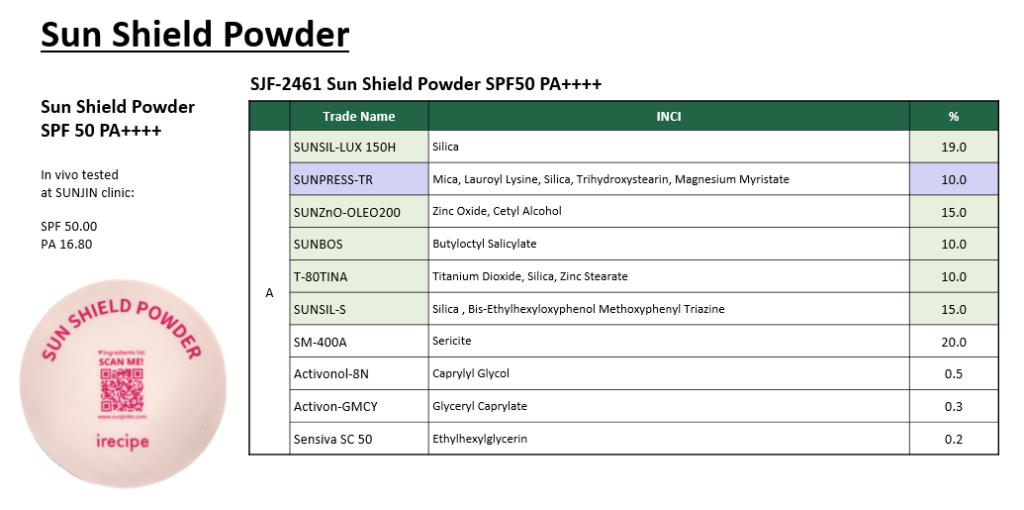
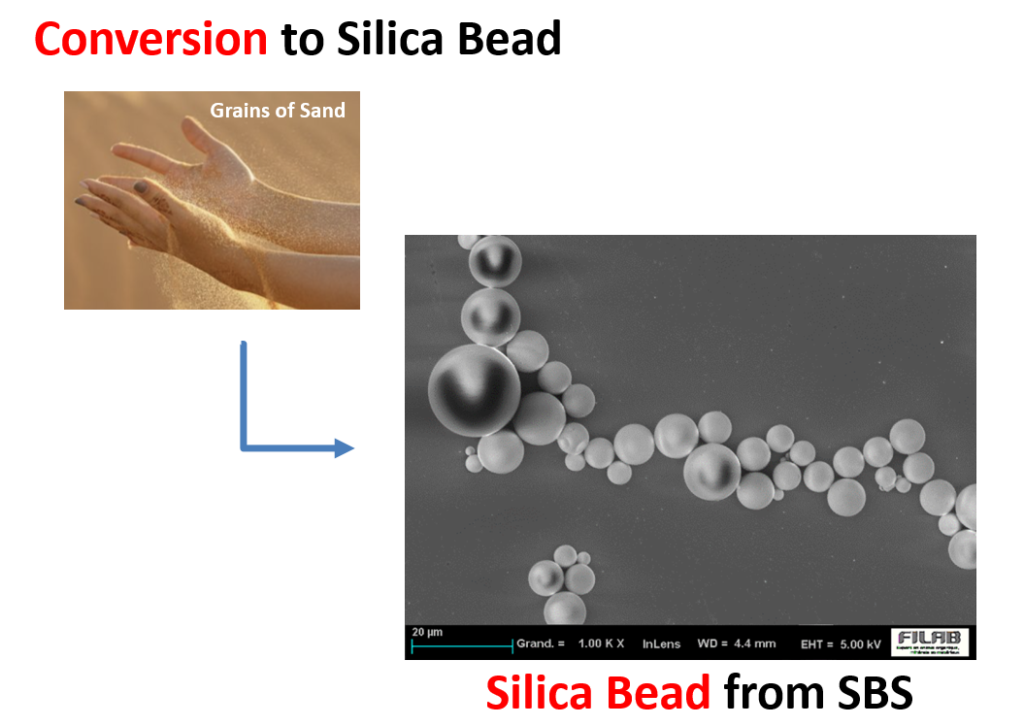
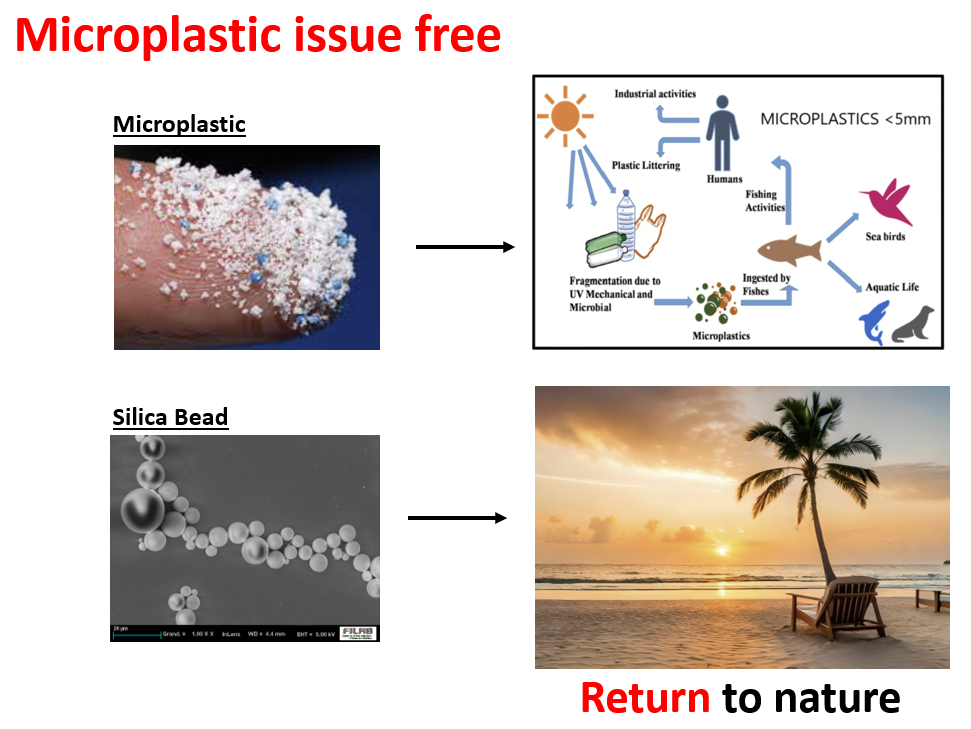
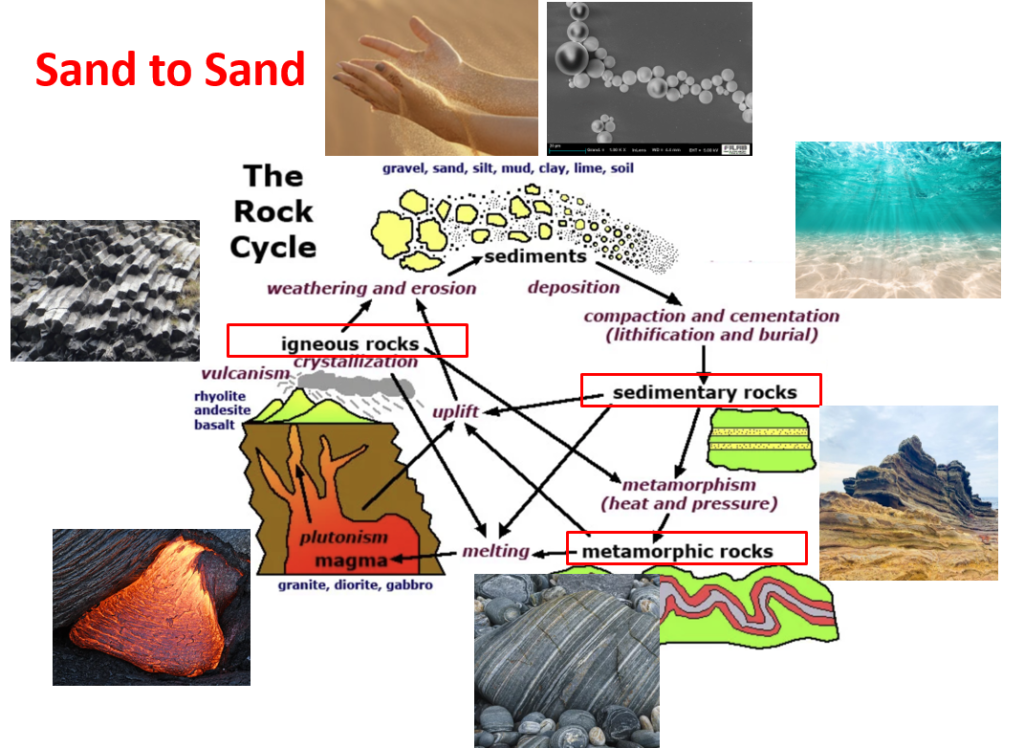
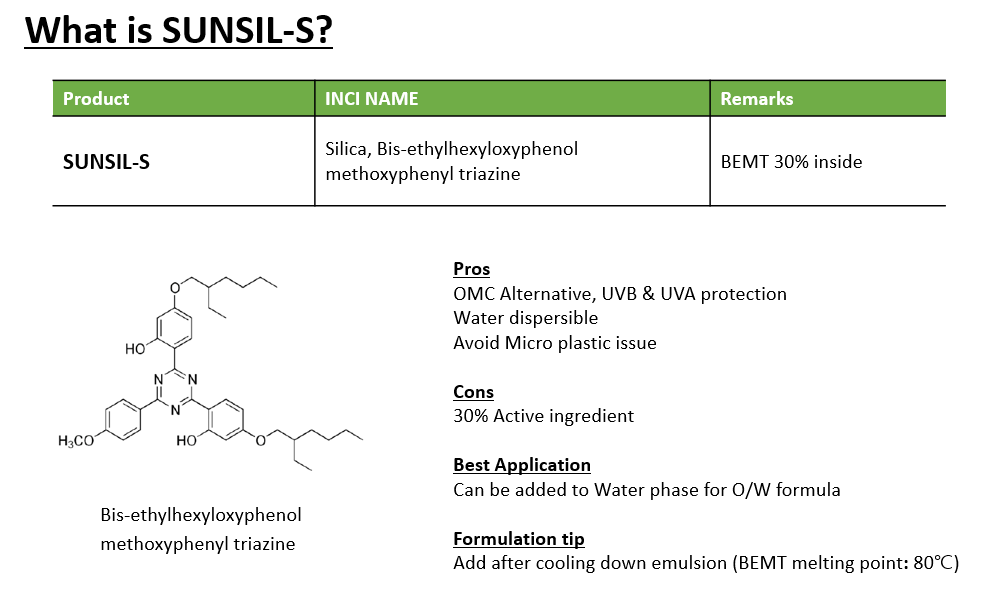
SUNJIN NEWSLETTER
EZLite-SF, Enzyme Oil with a Light Feel and Fast Skin Absorption – Global Launch!
By Jeong, Areum
Translated By Ko, Eunyoung
July 2024, NO.31
Hi, How are you?
We are excited to introduce EZLite-SF, a sunflower oil-based enzyme oil characterized by its light feel and rapid skin absorption, made possible through bioconversion technology.

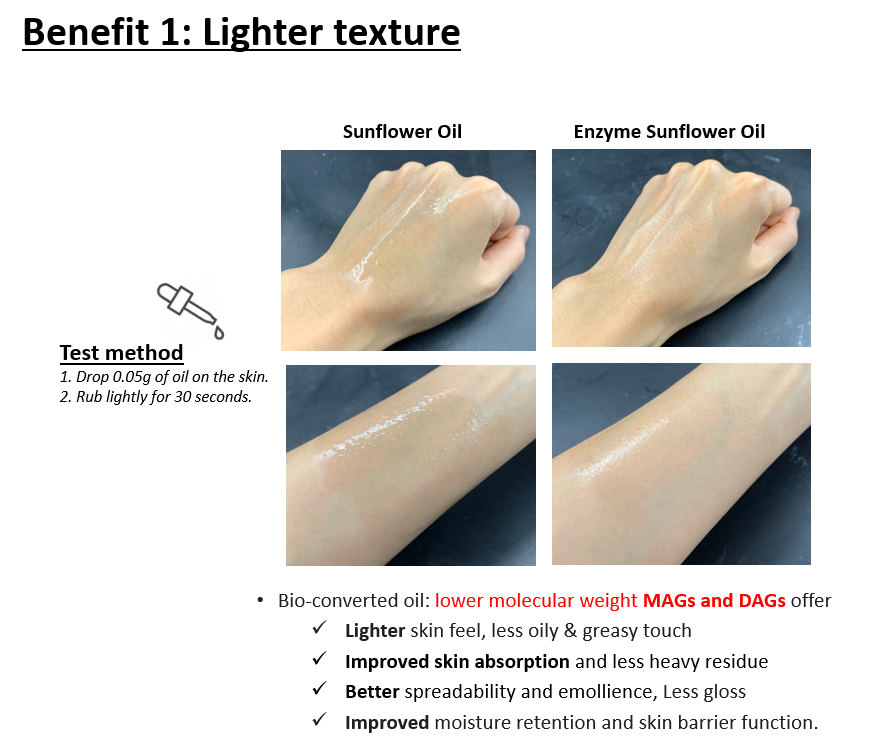
Enzyme oil is a product that leverages bioconversion technology to adjust the ratios of MAG, DAG, and TAG in traditional vegetable oils. By reducing the proportion of larger molecular weight TAGs and increasing the proportions of smaller molecular weight MAGs and DAGs, EZLite-SF offers a lighter feel and faster skin absorption compared to conventional vegetable oils.
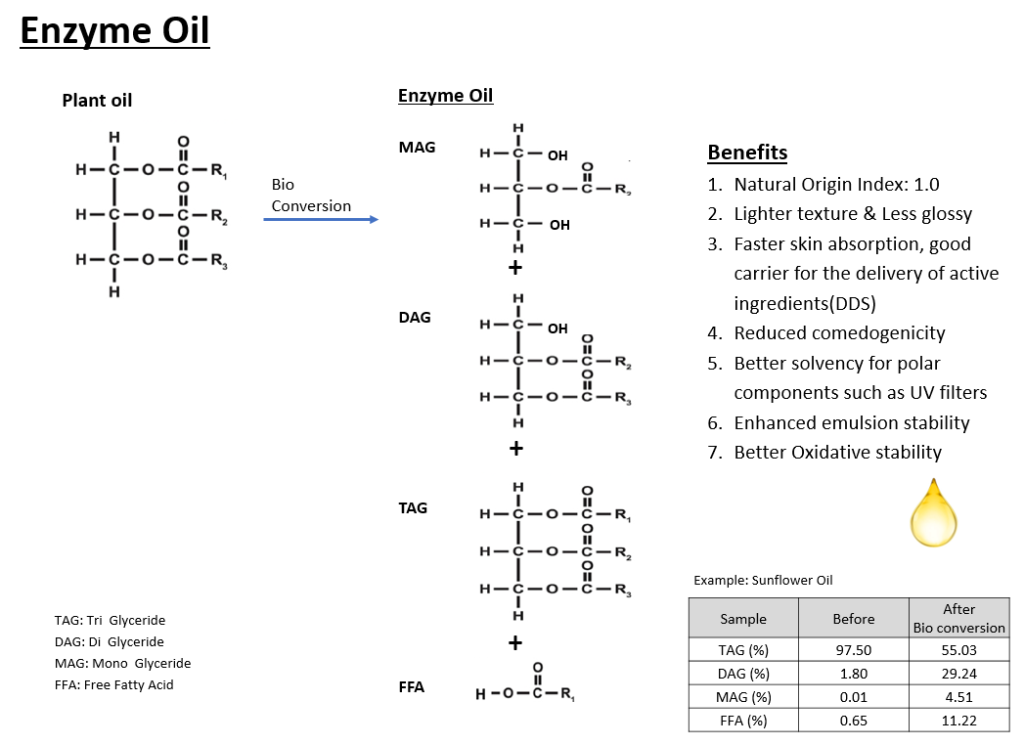
Comparative analysis between traditional sunflower oil and the enzyme oil EZLite-SF has shown that EZLite-SF, with its higher ratio of low molecular weight MAGs and DAGs, is less greasy and absorbs more quickly.
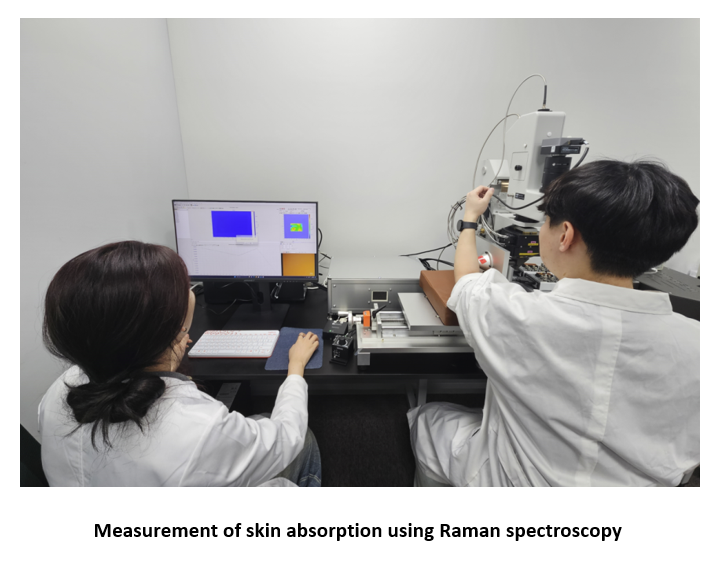
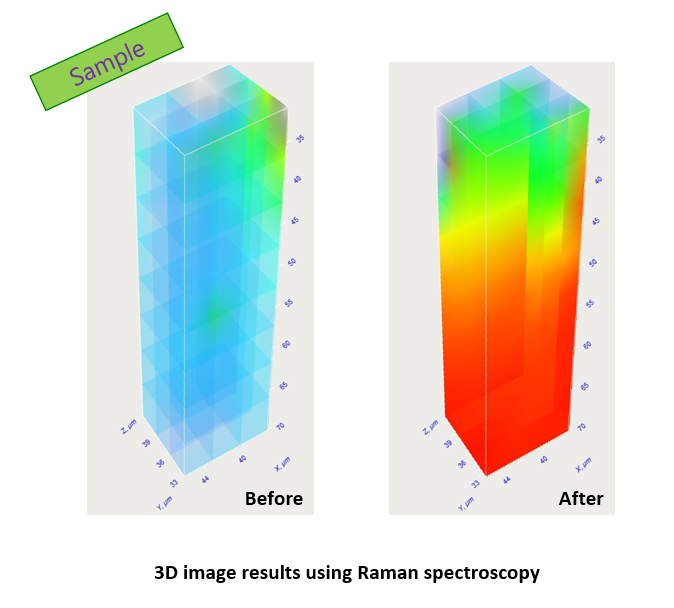
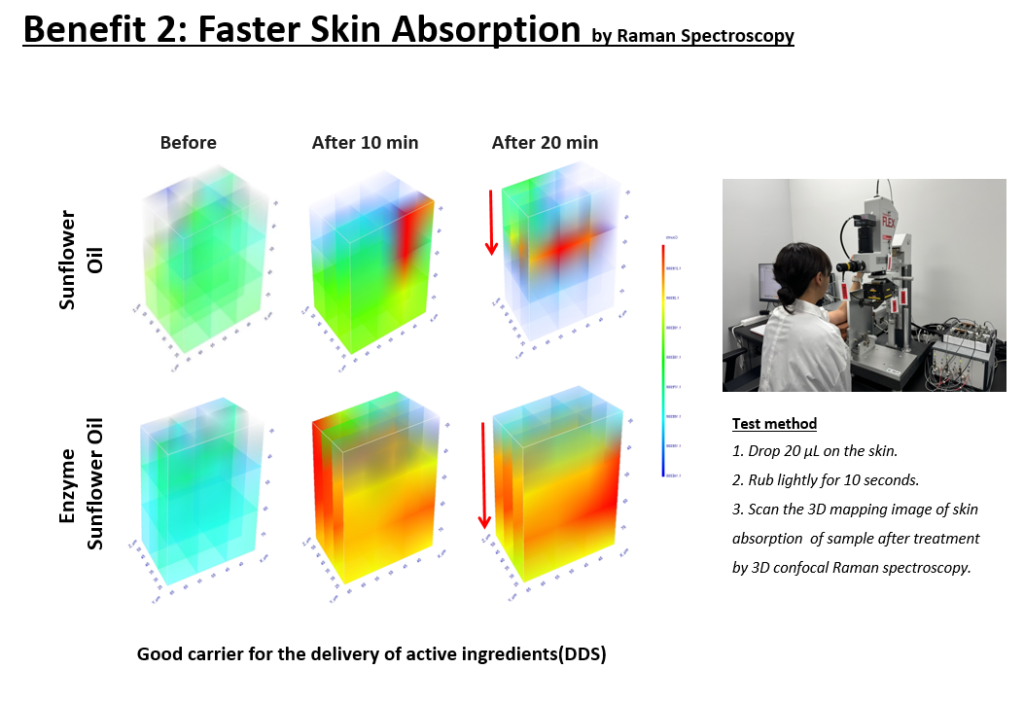
This accelerated skin absorption has been confirmed through Raman Spectroscopy, where measurements taken before application and at 10 and 20 minutes post-application reveal significantly faster absorption of the enzyme oil over time.
Additionally, enzyme oil demonstrates superior solubility for polar ingredients such as UV filters and ceramides, enhancing emulsion stability among other benefits.
Currently, only EZLite-SF, derived from sunflower oil, is available, but we plan to expand this product line in the future. We appreciate your interest and support.
If you have any inquiries, please feel free to contact us
Areum Jeong / Regional Sales Manager
E-mail : sales1@sunjinbs.com