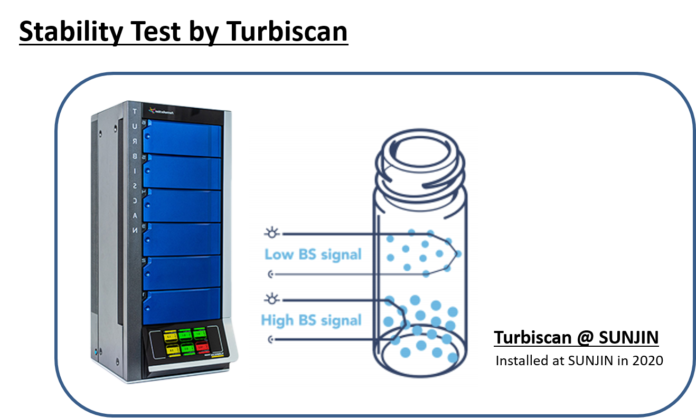
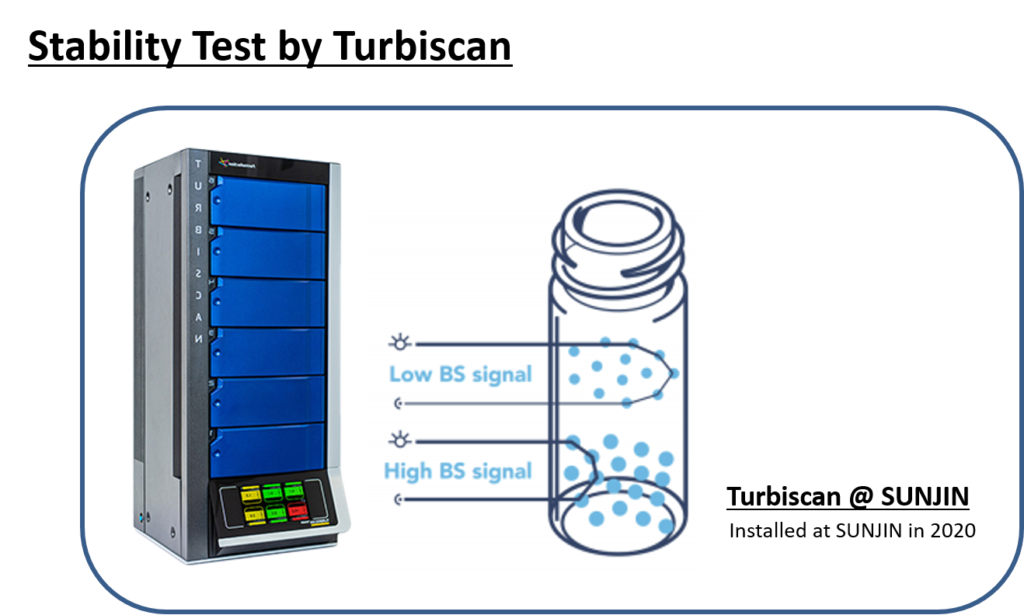
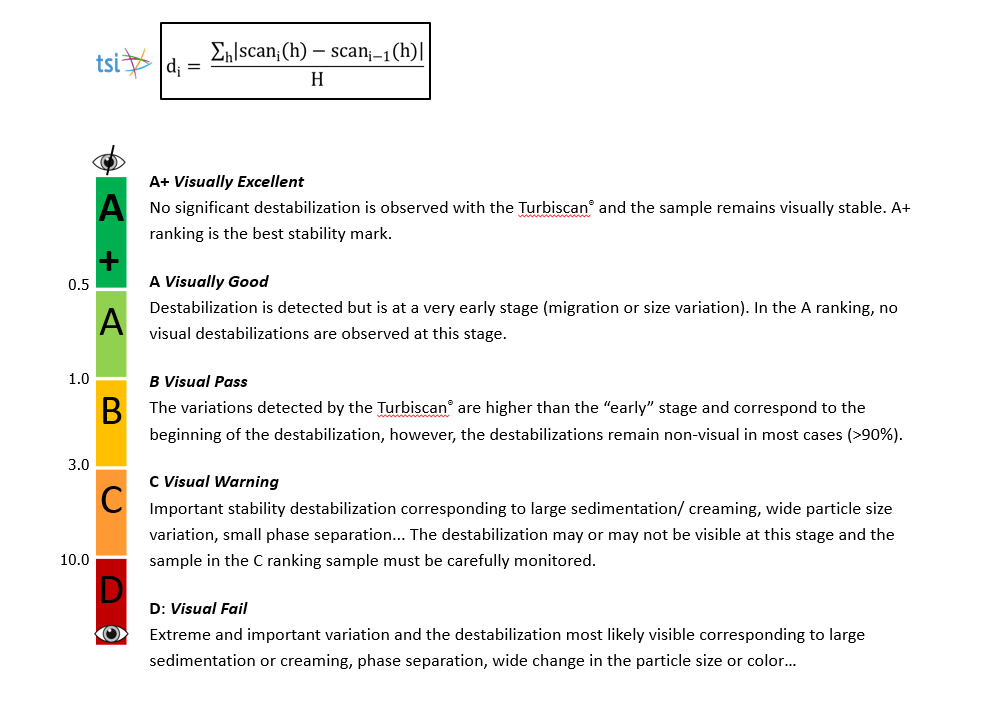
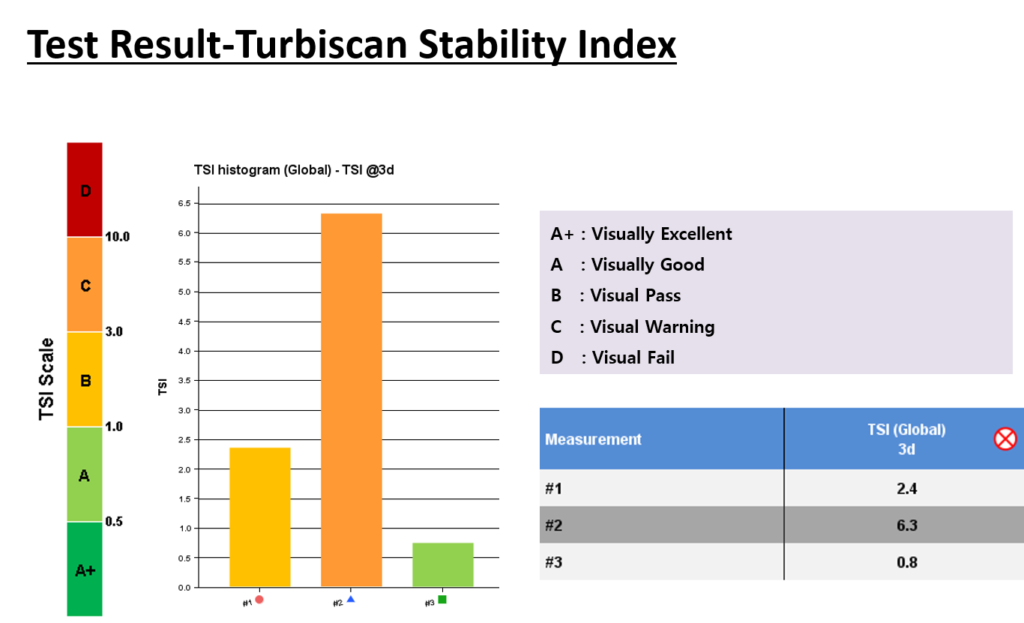
SUNJIN NEWSLETTER
Invitation to Sunjin Booth at In-Cosmetics Korea 2021
By Kim, Sanguk
July 2021, NO.22
Hi, How are you?
In-Cosmetics Korea 2021 will be held at COEX Convention Center, Seoul from 14th to 16th July. Sunjin will participate and introduce new ingredients and formulations.

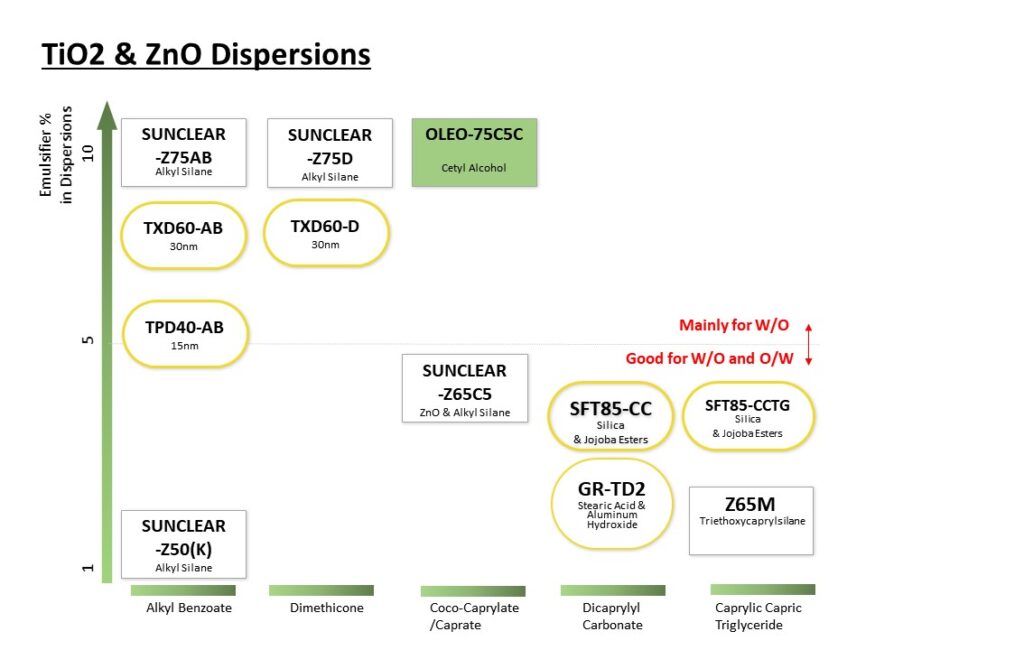
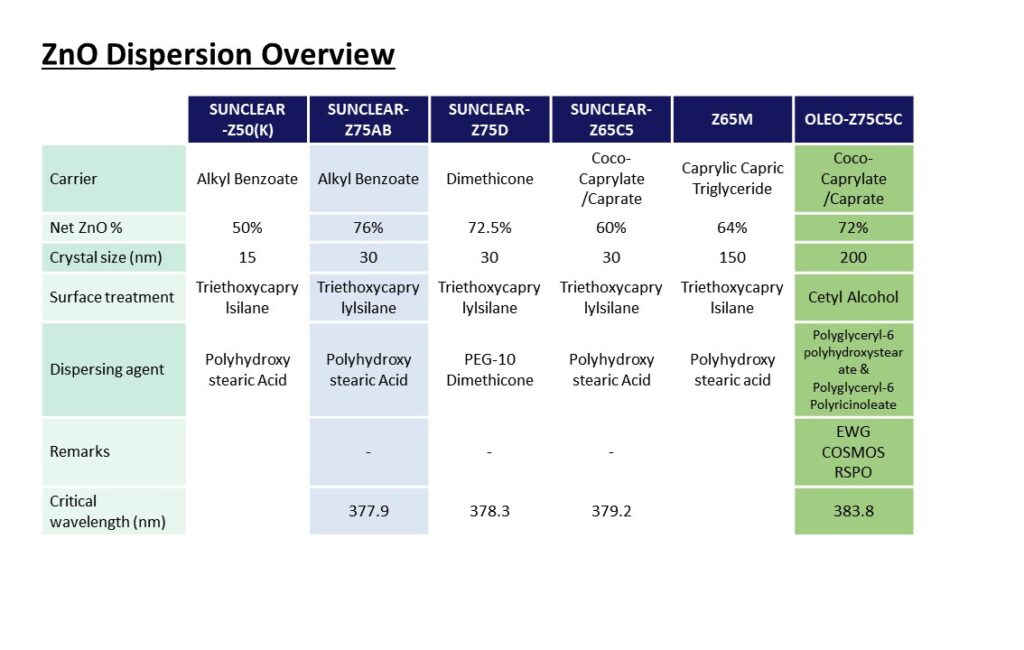
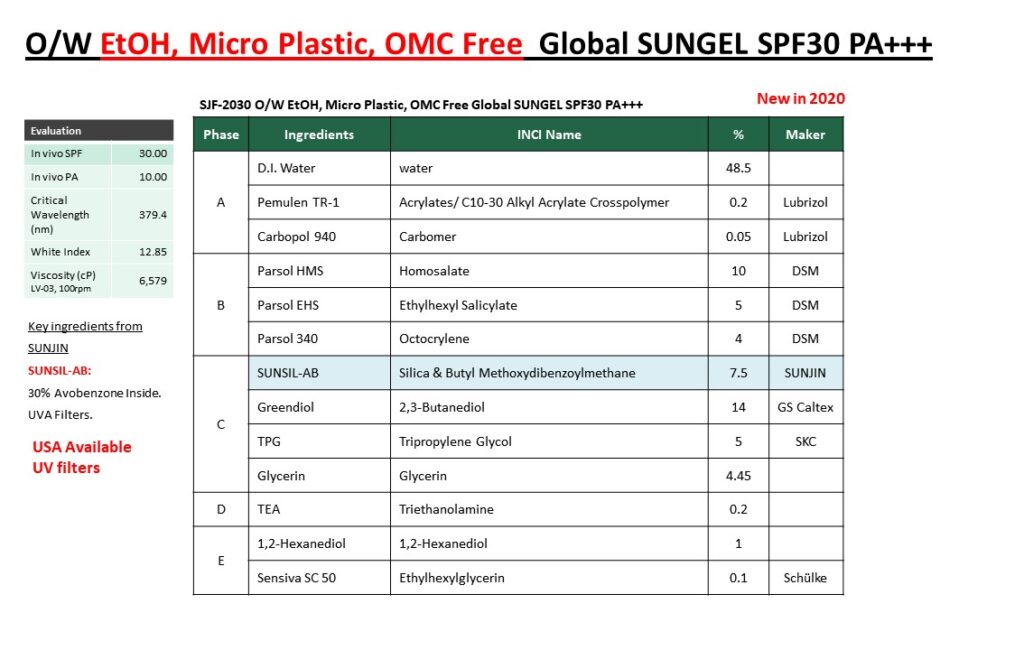
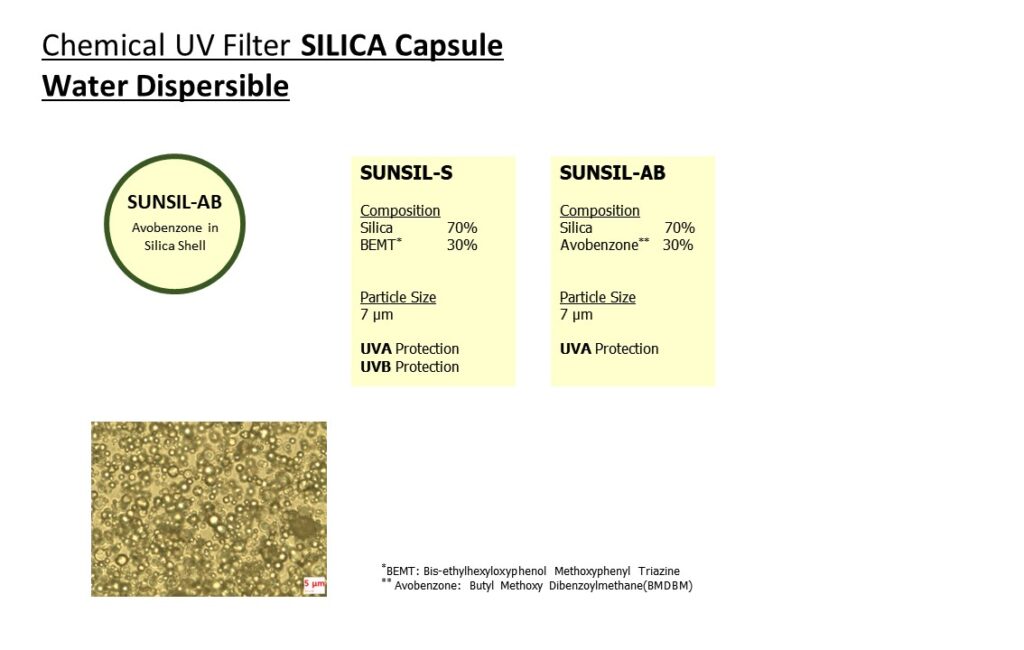
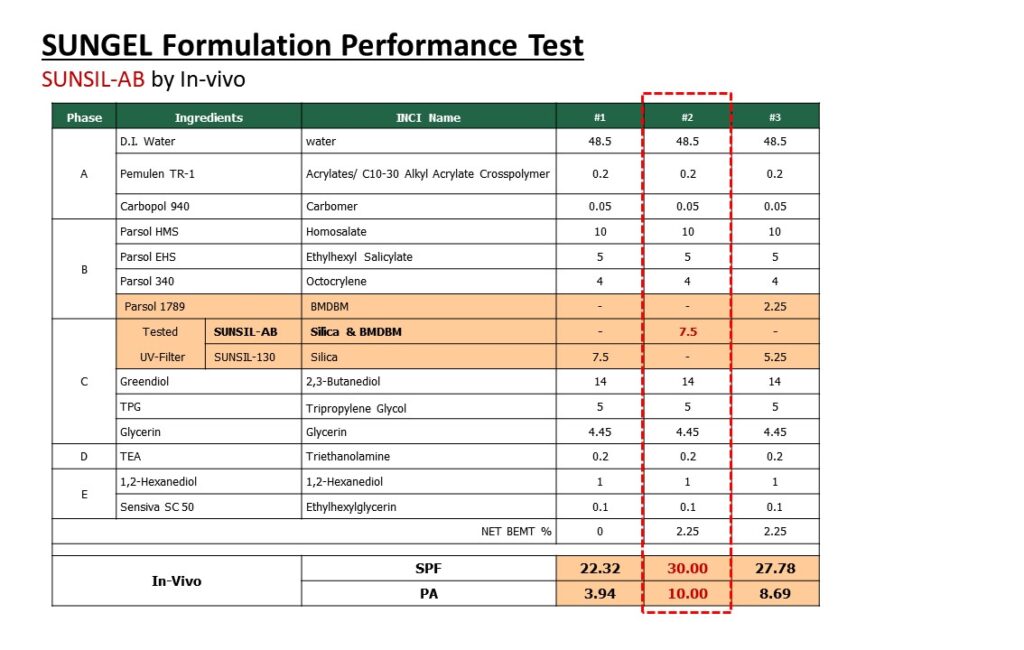
Please visit our booth if you would like to learn how to formulate foundation make-up to achieve UV Protection, Coverage and Dark shades at the same time.
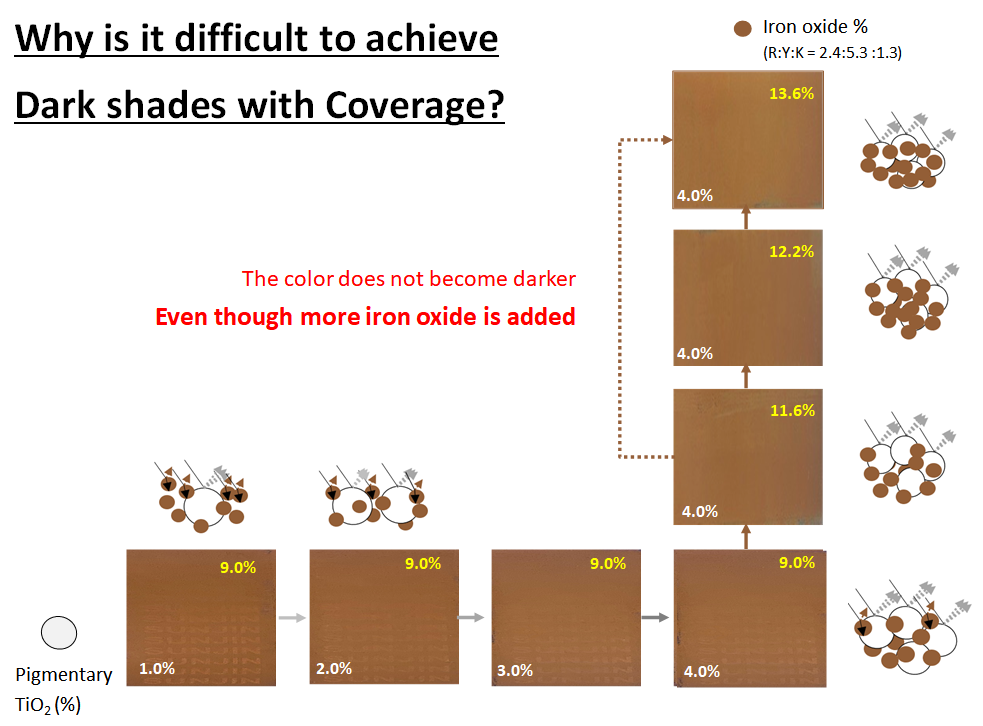
Not only that, please come see our various ingredients and formulations.
• When: 14th–16th July 2021
• Where: Hall C in COEX Convention Center, Seoul
✓ SUNJIN Booth: D10
Please contact us for more information.
Areum Jeong
E-mail : sales1@sunjinbs.com
Tel : +82 31 364 0721