SUNJIN NEWSLETTER
The secret method to pass the FDA Inspection
By Kim, Sanguk
May 2021, NO.15
Hi, How are you?
We would like to share how Sunjin passed the FDA inspection with No Action Indicated(NAI) as the first cosmetic raw material manufacturer in Korea.

First, we have invested heavily in the construction of Top Down, Closed Production Line. This system makes it possible to prevent microbiological contamination and cross contamination, which is one of the FDA requirements.
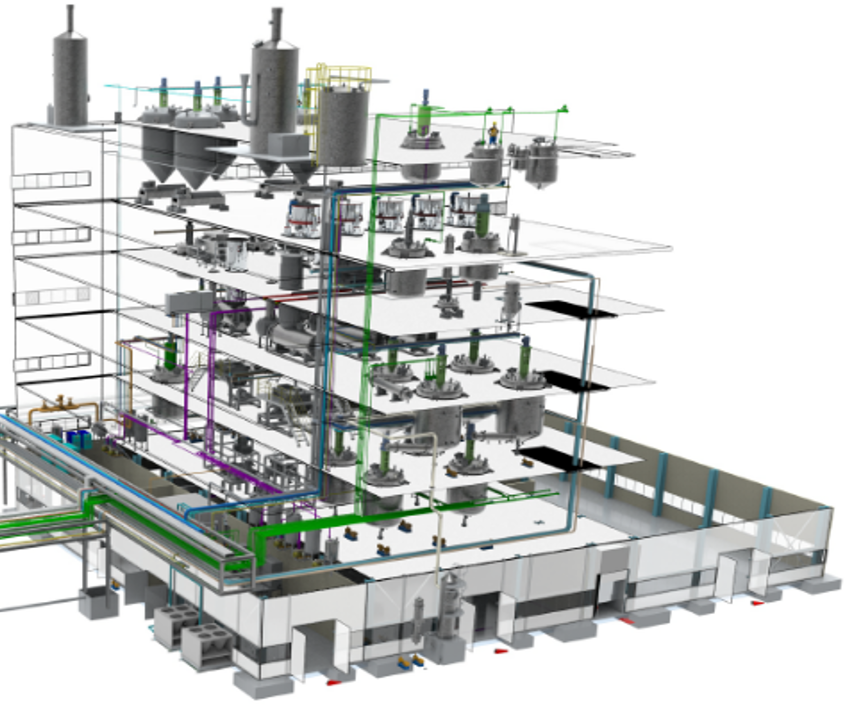
This Smart Factory is our own design. Large capital expenditure was required to build the automatic and dedicated production line optimized to Active Pharmaceutical Ingredient(API) production.

In addition, we recruited many QA, QC staffs with pharmaceutical backgrounds to enhance the capability of quality management. In order to meet the FDA requirement on Data Integrity, we educate and train production staffs and prevent tamper problem on records.

If you have any inquiries about FDA Inspection, please contact us.
Areum Jeong
E-mail : sales1@sunjinbs.com
Tel : +82 31 364 0721

