SUNJIN NEWSLETTER
Thai FDA Strengthens Safety Standards through TiO2 Cosmetic Regulations!
By Kim, Yongsung
Translated by Lee, James
December 2024, NO.53
Hi, how are you?
On November 18, 2024, the Thai Food and Drug Administration(Thai FDA) revised regulations regarding the use of titanium dioxide in cosmetics, modifying restrictions, permitted pigments, and requirements for use as a UV filter.

This amendment reflects the approvals from the 39th ASEAN Cosmetic Scientific Body(ACSB) conference held in July 2024, aiming to align Thailand’s cosmetic regulations with the latest ASEAN Cosmetic Directive(ACD).


Under this amendment, titanium dioxide can be used in cosmetics only under specific conditions, with precise restrictions on concentration and product types.
For example, titanium dioxide can be used as a UV filter, but in nano form, it must meet specific safety requirements. Additionally, new labeling requirements are being applied to products containing titanium dioxide to ensure clear information for consumers.
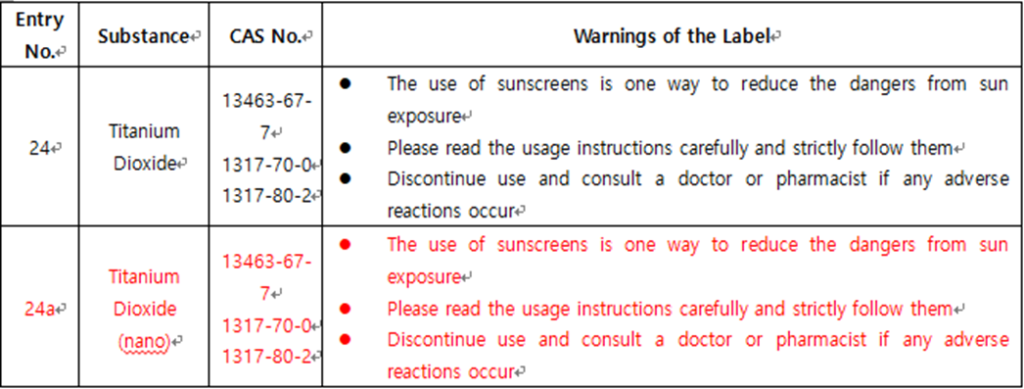

This regulatory amendment is part of Thailand’s efforts as an ASEAN member state to adhere to regional standards, enhance consumer safety, and improve the international competitiveness of the cosmetics industry.
Cosmetic manufacturers and importers must be aware of these changes and ensure their products comply with the new regulations.

To provide customers with trustworthy products, SUNJIN has conducted aerodynamic diameter analysis of our titanium dioxide through an external professional analytical institution.
The analysis results show that our titanium dioxide products contain less than 1% of particles with an aerodynamic diameter over 10µm, thereby perfectly meeting the latest regulations required by Thai FDA.
SUNJIN continuously monitors cosmetic industry regulations announced by regulatory agencies in the United States, Europe, and other countries, supporting our customers in ensuring their products comply with regulations across different markets.
We remain committed to providing products that customers can trust through ongoing quality management and consistent regulatory compliance efforts.
For inquiries about related products and formulations, please feel free to contact us using the information below.
Areum Jeong / Regional Sales Manager
E-mail : sales1@sunjinbs.com


