SUNJIN NEWSLETTER
IARC Raises Talc’s Cancer Risk Classification
By Kim, Yongsung
Translated By Ko, Eunyoung
September 2024, NO.40
Hi, how are you?
In this issue, we would like to introduce the recent announcement from the International Agency for Research on Cancer (IARC) regarding the updated cancer risk classification of talc.

Last July, the IARC, an agency under the World Health Organization (WHO), published a reassessment of the carcinogenic potential of talc and acrylonitrile in the British medical journal Lancet Oncology.

Talc, a mineral substance widely used in cosmetics, food, pharmaceuticals, and other consumer goods, has long been subject to concerns about its potential carcinogenicity.
According to this new study, even talc that does not contain asbestos or asbestos-like substances is now classified as probably carcinogenic to humans.
•Group 2A: Probably carcinogenic to humans
•Group 2B: Possibly carcinogenic to humans

The reclassification of talc’s cancer risk is expected to result in regulatory impacts, such as usage restrictions or warning labels under California Proposition 65 in the United States and the European Cosmetics Regulation EC 1223/2009.

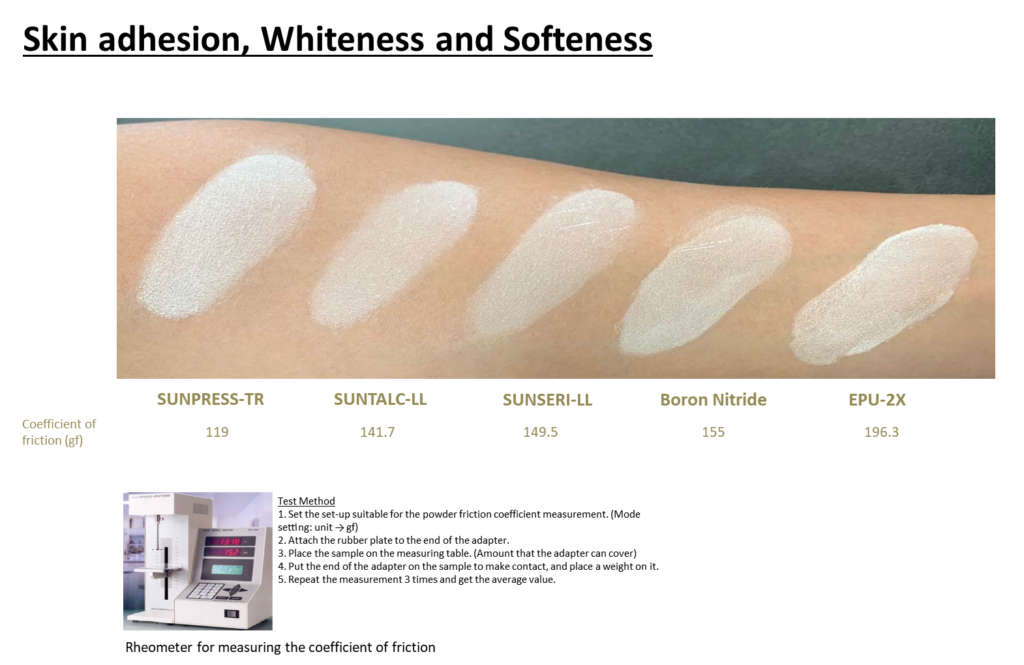
In response, Sunjin introduces SUNPRESS-TR as a substitute for talc, which has been newly classified with an increased cancer risk.
This raw material spreads more smoothly with a lower coefficient of friction than talc, providing a gentle, natural adherence to the skin without irritation.
Additionally, with excellent molding properties, it stays undamaged during drop tests, ensuring superior product quality and durability compared to talc.
Experience a safer and more innovative alternative to talc with this new material.
If you have any inquiries, please feel free to contact us.
Areum Jeong / Regional Sales Manager
E-mail : sales1@sunjinbs.com


