SUNJIN NEWSLETTER
Sunjin responds to the new China NMPA regulations
By Kim, Sanguk
February 2022, NO.05
Hi, How are you?
Today we would like to explain the new China NMPA(formerly the China Food & Drug Administration or CFDA) regulations and how Sunjin is responding to the new regulations.

In China, 「Measures for the Administration of the Registration and Recordation of Cosmetics」 was formulated in order to establish a database for safety evaluation and product traceability. Therefore information on all raw materials in a formulation will be open, and safety data must be registered in NMPA’s “cosmetic ingredients safety information registration platform”
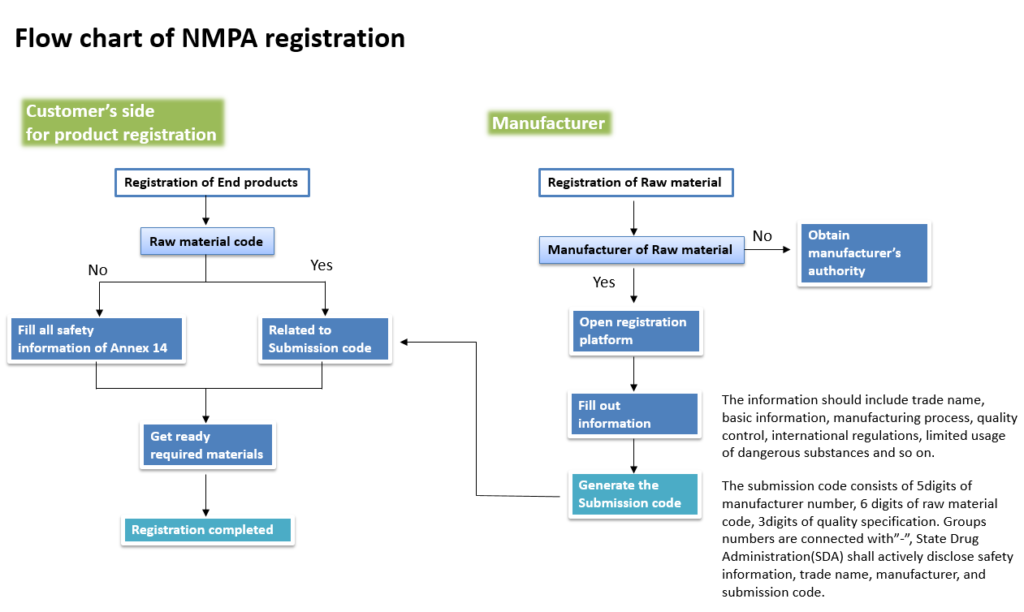
Sunjin will register our raw materials on the NMPA platform and provide “Submission Code” to customers. Through this, customers can easily access product information by entering the “Submission Code” on the NMPA platform without having to register separately by receiving safety-related information on all raw materials in product formulations.

The NMPA platform opened on December 30th, 2021. Starting with SUNZnO-NAS, Sunjin is registering raw materials on the platform. We promise to proceed registration in sequence and provide submission code for all of our products.
If you have any inquiries, feel free to contact us.
Areum Jeong / Regional Sales Manager
E-mail : sales1@sunjinbs.com

