SUNJIN NEWSLETTER
What is NDC?
By Kim, Sanguk
March 2021, NO.5
Hi, How are you?
Have you heard of NDC? NDC stands for National Drug Code. It is a unique product identifier used in the U.S. for drugs.
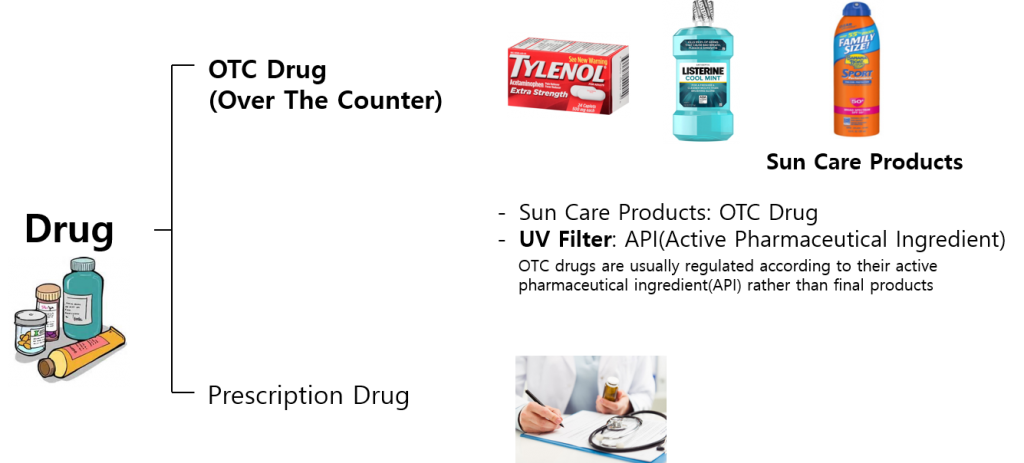
Sunscreens are OTC drugs which are sold directly to a consumer without a prescription. UV filters in sunscreens are considered as active pharmaceutical ingredients(API) and they are strictly managed by the FDA.
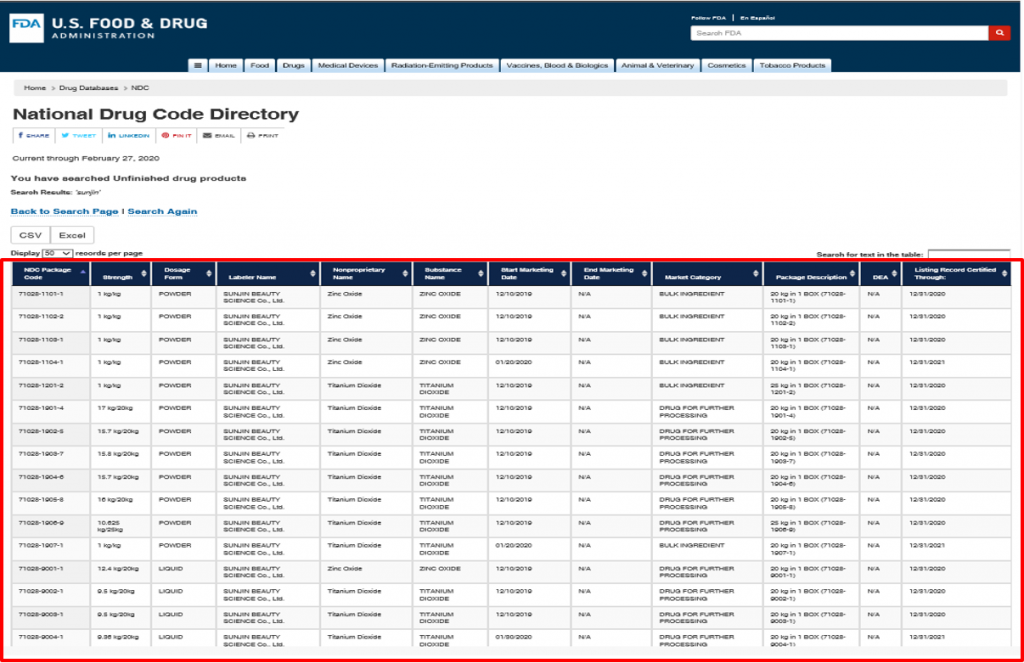
Therefore, the NDC code is required to sell UV filters in the U.S. market.
SUNJIN provides various NDC registered UV filters. More details can be found in the following links. Please visit the sites if you are interested.
<Relevant information>
– NDC Directory
https://www.accessdata.fda.gov/scripts/cder/ndc/dsp_searchresult.cfm
– U.S FDA Drug Definitions_Registrar Corp
https://www.registrarcorp.com/definitions/
– What must I do to import a human drug product that has been approved by the FDA into the US?_FDA U.S Food&Drug
https://www.fda.gov/industry/fda-basics-industry/what-must-i-do-import-human-drug-product-has-been-approved-fda-us

