| |
| |
| SUNJIN NEWSLETTER |
|
Updates to the clinical test service from ISO24444 revision
|
by Sung-Ho Lee
April 2020, NO.241 | |
| |
Dear everyone,
SUNJIN is now providing “free clinical preliminary test” for sun protection with new ISO24444
edited last December.
|
| |
 |
| |
|
In edited version, they revised the definition of Minimal Erythema Does (MED), selection of
participants, application procedures, reference standard sunscreens, evaluation method and test
reports were provided. Please see more details on the changes made in the edited version through
following pages.
|
| |
 |
|
| |
|
| |
|
1. The definition of the minimal erythema dose (MED)
|
| |
| Before |
After |
 |
 |
MED (minimal erythema dose)
: First noticeable erythema found with minimal
UV
radiation.
|
MED (minimal erythema dose)
: 50 % of erythema found with minimal UV
radiation. |
|
| |
|
| 2. The selection of participants |
| |
| Before |
After |
 |
 |
| |
Standard for the selection of participants
: Participants need to have ITA° value above 28° and
they can be tested for skin type I, II, III.
|
| |
| |
55 < ITA value Type 1
41 < ITA value Type 2
28 < ITA value Type 3
10 < ITA value Type 4
|
 |
SPF is Measureable
PA is Measureable |
|
| |
|
|
| |
Standard for the selection of participants
: Average of ITA° value for total participants need to be
between 41°~ 55° with average skin type of II. |
| |
| |
55 < ITA value Type 1
41 < ITA value Type 2
28 < ITA value Type 3
10 < ITA value Type 4
|
 |
SPF is Measureable
PA is Measureable |
|
| |
|
|
|
| |
|
| 3. The additional standard samples for SPF measurement |
| |
| Before |
After |
 |
 |
| Estimated Value |
|
Reference sunscreen
formulation |
|
|
|
| SPF < 20 |
|
P2, P3, P7 |
|
|
|
| SPF ≥ 20 |
|
P2, P3 |
|
| Estimated Value |
|
Reference sunscreen
formulation |
|
|
|
| SPF ≤ 24 |
|
P2, P3 |
|
|
|
| 25 < SPF ≤ 5 0 |
|
P2, P3, P5, P6 |
|
|
|
| 50 ≤ SPF |
|
P2, P3, P8 |
|
| |
Previously, only P2, P3, and P7 standard samples were available to measure below or above SPF 20, but now P5, P6,
and P8 standard samples are added in order to measure more precisely for the samples above SPF 25.
|
|
|
| |
|
| 4. The application procedures |
| |
| After |
|
| |
Previously, application methods were only available for liquid and powder types, but now they provided application
method for various types of samples including Lotion, Cream, Liquid, Stick, Spray, and Powder with appropriate
flow chart. |
|
|
| |
|
| 5. The evaluation methods |
| |
| After |
|
| Example |
 |
| |
They classified with different grades depend on the level of erythema formed and they provided appropriate
evaluation methods for each different grade with examples of each. In addition standardized for rejection as well.
|
|
|
| |
|
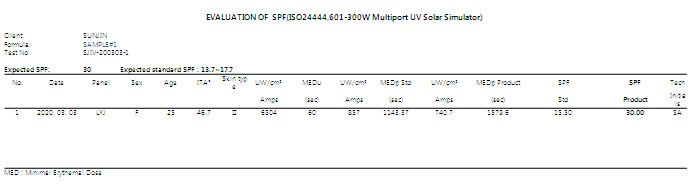
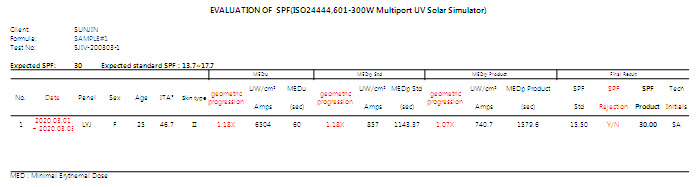
| 6. Additional data for a test report
|
| |
| Before |
 |
| After |
 |
| |
Test report will be including intensity increment value and following reason for a rejection. |
|
|
|
| |
 |
|
| |
| Free clinical test service by SUNJIN |
| |
|
SUNJIN provides free clinical test service for anyone who uses SUNJIN materials in their formulation
so please feel free to use the service.
|
| |
| Free education on SPF/PA clinical test |
|
|
| |
 |
| |
|
| Free UV In-vivo/ In-vitro clinical test |
|
|
| |
 |
|
| Service Features |
|
 |
| 1. Free |
| SUNJIN customers can apply the service for free |
|
 |
| 2. Fast |
| Generally it takes 2~3 weeks to receive results |
|
 |
| 3. High reliability |
More than 700 cases per a year
Annually calibrate results with external agencies |
|
|
|
|
|
| |
 |
|
| |
| If you have any concerns or question on our clinical test service, please contact through following
information. Thank you. |
|
 Areum Jeong Areum Jeong |
– E-mail : sales1@sunjinbs.com
– Tel : +82 31 364 0721 |
| |
|
|
| |
| |