SUNJIN NEWSLETTER
UV Filters with FDA NDC codes by Sunjin
By Kim, Sanguk
August 2021, NO.26
Hi, How are you?
Today we would like to introduce Sunjin’s products with NDC code. The NDC code for UV filter is required by the FDA to sell a sunscreen which is an OTC(Over-the-counter) drug in the U.S. market.

As the first manufacturer to pass FDA inspection in Korea, Sunjin has NDC(National Drug Code) codes for the UV filters.

The products with NDC codes are TiO2 and ZnO powders and dispersions, as you can see the table below.
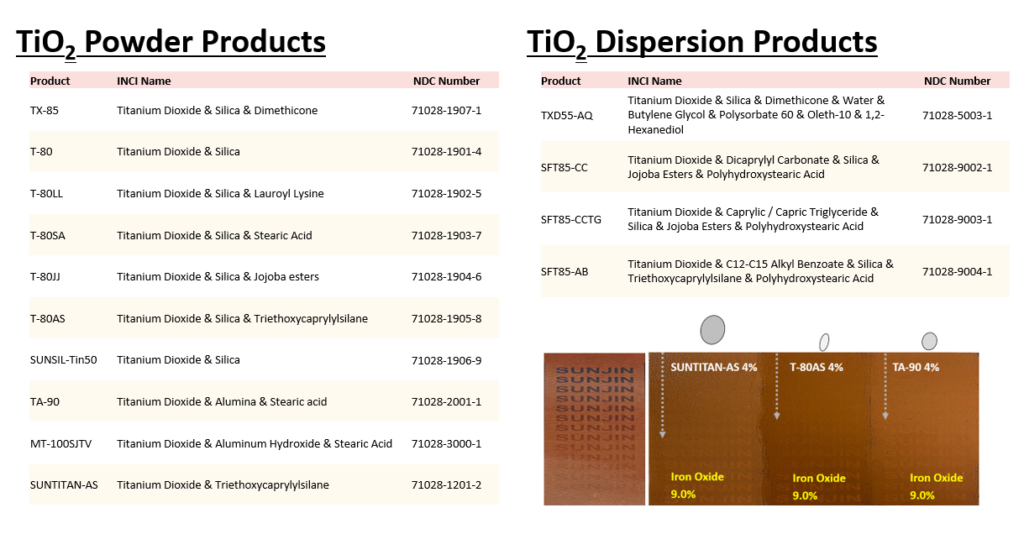
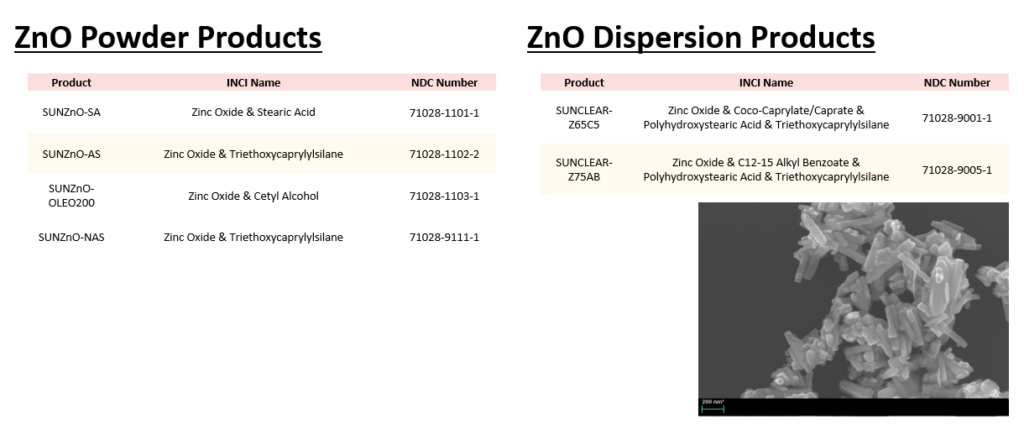
If you are interested in UV filter products with NDC code, feel free to contact us.
Areum Jeong
E-mail : sales1@sunjinbs.com
Tel : +82 31 364 0721

